National Institutes of Health
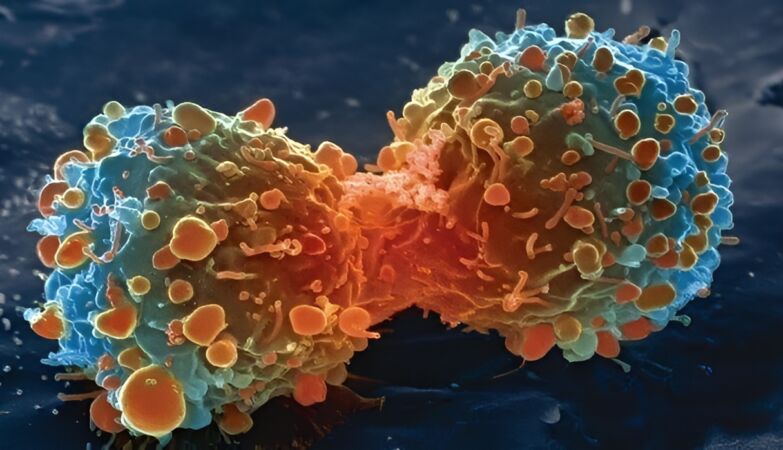
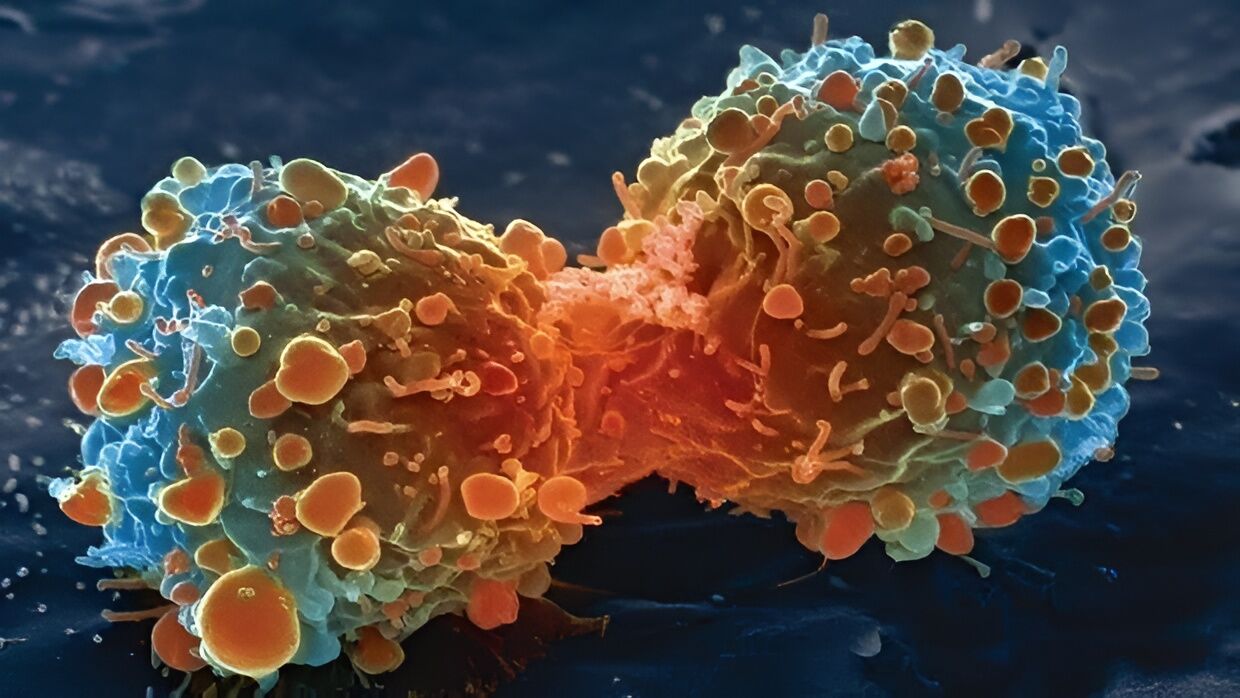
Lung cancer cell during cell division
Aging brings two opposing trends in cancer risk: First, it increases in our 60s and 70s, as decades of genetic mutations accumulate in our bodies. But then, around age 80, it slows again — and a new study may explain a key reason why.
In a new study, the results of which were presented in an article published this month in Naturean international team of scientists analyzed lung cancer in mice, monitoring the behavior of type 2 alveolar stem cells (AT2).
These cells are crucial for lung regeneration and are also the starting point of many lung cancers.
What was found was that the levels of a protein called NUPR1 were higher in older mice.
This caused the cells to act as if they were iron deficientwhich, in turn, limited your regeneration rates – placing restrictions on both healthy growth and cancerous tumors.
“Aged cells have more ironbut, for reasons that we still don’t fully understand, they function as if they don’t have enough”, says the cancer biologist Xuejian Zhuangfrom Memorial Sloan Kettering Cancer Center (MSK), in New York, cited by .
“Aged cells lose their ability to renew and, consequently, the uncontrolled growth that occurs in cancer”, he adds.
It was found that the same processes were also happening in human cells: more NUPR1 leads to a drop in the amount of iron available to cells. When NUPR1 was artificially reduced or iron was artificially increased, the cells’ growth capabilities increased again.
This fact may provide researchers with a way to explore treatments that target iron metabolism, especially in older people. It could, for example, restore lung capacity of people suffering long-term effects from COVID-19.
These results also have implications for cancer treatments based on a type of cell death called ferroptosiswhich is triggered by iron. The researchers discovered that this cell death is less common in older cells, due to their functional iron deficiency.
Maybe that too make them more resistant to cancer treatments based on ferroptosis that are being developed, so the sooner a treatment against ferroptosis is tried, the the greater the probability to function.
“What our data suggests in terms of cancer prevention is that events that occur when we are young are probably very more dangerous than events that occur later“, says the cancer biologist Tuomas Tammelada MSK.
“That’s why, prevent young people from smokingtanning or other obvious cancer exposures are probably even more important than we thought,” the researcher adds.
There is much more to explore about the effects of NUPR1 and its relationship to stem cell function – both healthy regeneration and cancerous growth – but these are important discoveries for fighting cancer at any stage of life.
As always with cancer treatments, it is necessary to take into account several factors: the type and stage of the cancerother medical conditions that may be involved, and (as this new study shows) the individual’s age. The more personalized the treatments, the more effective they will be.
“But in reality, not much is known yet about how aging changes cancer biology”, concludes Zhuang.