By combining photochemical reactions with molecular self-assembly, scientists have achieved the impossible: using light to create molecular adjustments that challenge thermodynamic equilibrium.
An innovative approach led in a new study, which takes advantage of light for molecular manipulationcould revolutionize technology and medicine.
Using a creative combination of light-induced photochemical reactions and molecular self-assembly, the research team used sunlight to develop innovative materials, smart medicines and dynamic systems that imitate non-equilibrium processes two living organisms.
The team, led by Alberto Crediprofessor at the University of Bologna, managed to insert a thread-shaped molecule into the cavity of a ring-shaped molecule, forming a high energy structure qthat would normally be impossible in thermodynamic equilibrium.
Essentially, light allows creation of molecular configurations that to nature cannot achieve by itself.
“We have shown that the administration of light energy to an aqueous solution prevents a molecular self-assembly reaction reaches a minimum thermodynamic, giving rise to a distribution of products that does not correspond to that observed in equilibrium”, says Alberto Credi, in a statement published on .
“This behavior, which is at the origin of many functions of living organisms, is little explored in artificial molecules because It’s very difficult to plan and observe”, adds the Italian researcher.
“A simplicity and versatility Our approach, together with the fact that visible light — that is, sunlight — is a clean and sustainable source of energy, allows us to predict developments in several areas of technology and medicine”, concludes the researcher.
Self-assembly: The heart of nanotechnology
The self-assembly of molecular components to obtain systems and materials with nanoscale structures (1 nanometer = 1 billionth of a meter) is one of the basic nanotechnology processes.
This process takes advantage of the tendency of molecules to evolve until they reach a state of thermodynamic equilibriumthat is, of minimum energy.
However, living beings function through chemical transformations that occur out of thermodynamic equilibrium and which can only occur through the supply of external energy.
Reproduce these mechanisms with artificial systems It’s a complex challenge and ambitious that could allow the creation of new substances, capable of responding to stimuli and interacting with the environment, which could be used to develop, e.g. smart medicines and active materials.
The interconnected components are the cyclodextrinswater-soluble hollow molecules with a truncated cone shape, and the azobenzene derivativesmolecules that change shape under the effect of light.
In water, interactions between these components lead to the formation of supramolecular complexes in which the filiform species of azobenzene is inserted by scientists in the cyclodextrin cavity.
Control of molecular orientation
In this study, the filiform compound has two different ends; Since the two rings of the cyclodextrin are also different, the insertion of the first into the second generates two distinct complexeswhich differ in the relative orientation of the two components.
University of Bologna
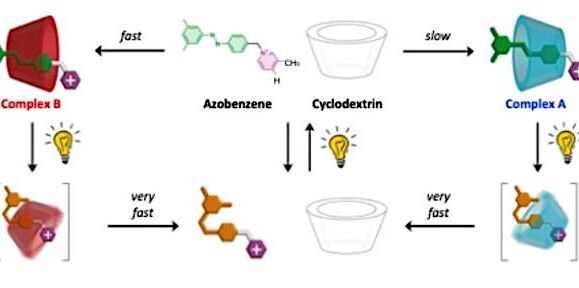
Schematic diagram of the self-assembly of cyclodextrin-azobenzene complexes in the dark (top of the figure) and under the action of light (full figure). The circles next to the complex structures represent the relative abundance of each complex in the dark (in black, equilibrium distribution) and under visible light irradiation with a wavelength of 453 nm (in yellow).
Complex A is more stable than complex Bbut the latter forms more quickly than the former. In the absence of light, only the thermodynamically favored complex, namely A, is observed at equilibrium.
By irradiating the solution with visible light, azobenzene changes configuration extended, similar to cyclodextrin, for a folded configurationincompatible with the cavity; as a result, the complex dissociates.
However, the same light can convert azobenzene back from the folded form to the extended form, and the dissociated components can get back together.
As complex B forms much faster than A, under continuous illumination it is reached a steady state where complex B is the dominant product.
When the light is turned off, azobenzene slowly reverses to the extended form and, after some time, only complex A is observed.
This self-assembly mechanism associated with a photochemical reaction allows harness light energy to accumulate unstable productsthus paving the way for new chemical synthesis methodologies and the development of dynamic molecular materials and devices (e.g., nanomotors) that function under non-equilibrium conditions, similar to those of living beings.









