Entities warn of risk of manipulated drugs for obesity and diabetes, highlighting lack of safety and effectiveness
The (Brazilian Society of Endocrinology and Metabology), the (Brazilian Society of Diabetes) and the (Brazilian Association for the Study of Obesity and Metabolic Syndrome) issued a note alerting about “Severe risks” associated with the use of injectable drugs of alternative origin or manipulated to treat obesity and diabetes.
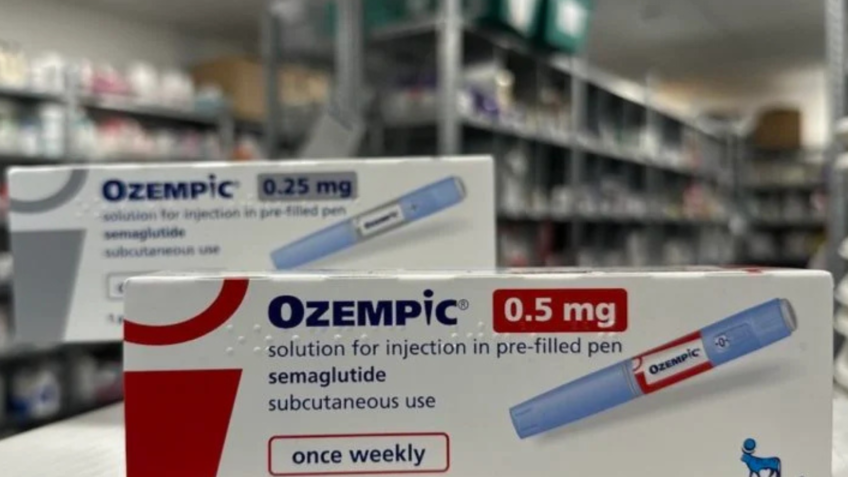
The drugs in question, according to the entities, are Analogs of GLP-1 and GIP, including Semaglutida (Ozepic and Wegovy, the pharmacist) and Tirzepatida (Mounjaro and Zepbound, from the laboratory), approved by regulatory agencies such as ( National Health Surveillance Agency) and the American (Food and Drug Administration).
“Biological medicines, such as semaglutide and tirzepatida, require rigorous manufacturing processes to ensure that the body uses and metabolizes the substance effectively and safely. These molecules are administered by subcutaneous injections, which requires rigorous patterns of sterility and thermal stability ”highlights the note.
The use of alternative or manipulated versions, according to the entities, figures as “Increasing, worrying and dangerous practice” and lacks scientific and regulatory bases that ensure product effectiveness, safety, purity and stability, “Exposing users to serious health risks, because they do not go through the necessary bioequivalence tests, making it impossible to predict their effects on the human body.”.
“FDA reports document serious administration problems in alternative or manipulated versions, with higher or lower doses than those recommended, contamination and replacement with other compounds”alert to note. “Alternative or manipulated semaglutado and tirzepatida are often disclosed as more affordable and equally effective options, which is a false promise.”states the text.
Also according to the entities, the direct commercialization of these alternative medications or manipulated by health professionals in offices configures practice contrary to the code of medical ethics, “Wreting the confidence of the doctor-patient relationship”. “Versions sold on websites, social networks or whatsapp also increase the risk of tampering, contamination and ineffectiveness by thermal destabilization”says the note.
Among the recommendations in the note is:
- that health professionals do not prescribe alternative or manipulated semaglute “They only use drugs approved by regulatory agencies, with certified industrial manufacturing and sold in pharmacies”;
- Patients reject treatments that include alternative or manipulated versions of these molecules – informing direct sales on websites, applications or offices – and seek alternatives approved by ANVISA;
- What regulatory and supervisory bodies, especially Anvisa and Medical Councils, intensify inspection actions on all companies and people involved in practice.
Finally, entities reinforce the importance of following recommendations based on science and ethics, prioritizing the safety and well-being of the population. “And they reiterate the social commitment to improve access to safe and effective medications to treat obesity and diabetes mellitus, as well as claim public policies for inclusion and broad approach to prevent and treatment of these conditions.”.
Falsification
In January 2024, the (World Health Organization) (World Health Organization) even warned the shortage of medicines as a global problem, especially in low and medium -income countries. According to the entity, since September 2021, the number of inputs missing in 2 or more countries has grown 101%.
“This shortage of medicines is a motive force recognized for falsified or lower quality medicines and entails the risk of many seeking medications through non -official means, such as the internet”highlighted the WHO, in a note.
The statement specifically cites global scarcity, registered in 2023, products indicated for the treatment of type 2 diabetes and also used for weight loss such as semaglutide. The substance is the active ingredient of Ozempic, a skin application pen for appetite control.
Anvisa
In October, Anvisa received a statement from Novo Nordisk pharmacist, responsible for the production of Ozempic, on evidence that insulin pens were readjusted and reused with labels of the drug. The suspicion was that the stickers were improperly removed from original pens from a batch of medication and used in insulin packaging sold.
Anvisa’s guidance is for the population and health professionals to be aware of the characteristics of Ozempic packaging and acquire only products within Caixa, in regularized pharmacies with health surveillance, always with the issuance of invoice.
The agency also recommends that no products and channel products are not purchased that sell medications using brands or sales applications, as well as social networking profiles that offer products.
With information from.