The decision was made after the record of 13 cases of adverse reactions between January 1 and March 19, 2025
Can your toothpaste be causing problems? Yesterday determined the interdiction of Colgate Clean Mint after several reports of adverse reactions.
What happened, what are the risks and what to do now? Let’s explain everything to you!
Famous toothpaste brand has product banned by Anvisa after reactions from customers
Colgate Clean Mint. (Photo: Disclosure)
The National Health Surveillance Agency (Anvisa) temporarily suspended the sale of Colgate Clean Mint.
The decision was made after the registration of 13 cases of adverse reactions between January 1 and March 19, 2025.
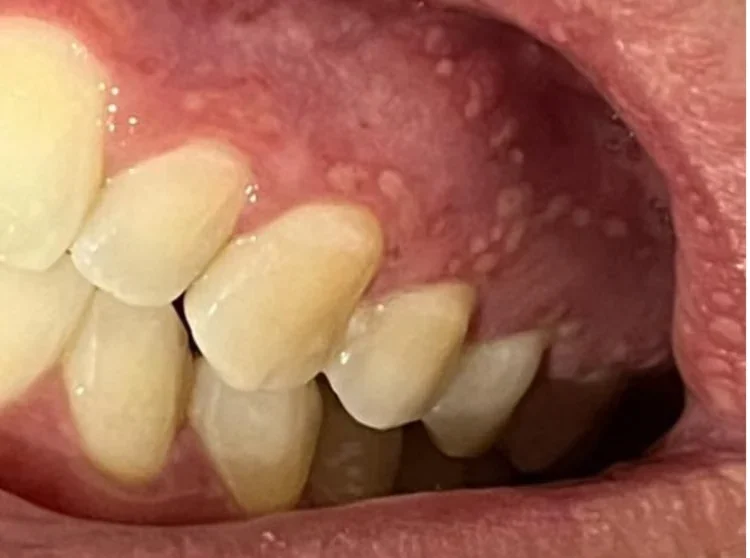
Consumers reported symptoms such as:
- Swelling of tonsils, lips and oral mucosa;
- Sensation of burning;
- Dormancy on the lips and mouth;
- Dry mouth;
- Irritated and red gum.
The interdiction of toothpaste has a period of 90 days, but Colgate may appeal the decision.
What has changed in the toothpaste formula?
The problem may be in the new composition. Colgate Total Active Prevention, launched in July 2024, replaced sodium fluoride with tin fluoride.
According to the company, the change is safe and based on over a decade of research and testing.
However, some people may be sensitive to tin fluoride or other components such as dyes and flavors.
If this occurs, Colgate recommends interrupting the use of the product as symptoms should disappear.
What does Colgate says?
The brand defended the safety of the new formula, stating that tin fluoride is widely used worldwide and approved by regulatory agencies.
However, he acknowledged that some people may have sensitivity to the ingredient.
In a statement, the company pointed out that all its products undergo strict testing and that consumers should interrupt use if they notice reactions.
What to do if you used the product?
If you are using Colgate Clean Mint and felt some symptoms, the ideal is to stop immediately. If symptoms persist, see a dentist or a doctor.
Meanwhile, the recommendation is to opt for other dental creams until ANVISA completes the investigation.
Stay tuned!
This interdiction is preventive and can be reversed. Still, it is important to follow up the case updates and choose products that guarantee your oral safety.