An alternative periodic table of the elements, focusing on highly loaded ions, reveals a new science that can support the demand for more accurate optical atomic watches.
According to, one New version of the periodic table of the elements predicted hundreds of highly loaded ions that could be used to create the Next generation of optical atomic watches.
A, invented by Dmitri Mendeleev In 1869, it groups the 118 chemical elements known according to their chemical properties. As the elements in the same parts of the periodic table share similar characteristics, this ordination allowed the chemicals Identify gaps in the table for decades And since then it has helped Discover the elements to fill them.
The table works very well for chemicals in general, but for some physicists, who are more interested in finding and using high energy ions, does not do the work they want. These particles are used in lasers X -rays, in plasma tumor therapy to test fundamental theories of physics and optical watches.
“We wanted to look for highly loaded ions for atomic watches, to make them much more stable and accurate,” he says Chunhai Lyufrom the Max Planck Institute of Nuclear Physics in Heidelberg, Germany.
Atoms are made up of a nucleus containing protons and neutrons, with electrons arranged in layers and sub -marked outside the nucleus. In an atom, there are an equal number of protons with positive charges and electrons with a negative charge. But atoms can win or lose electrons, forming loaded. An atom that loses many electrons becomes a highly charged ion.
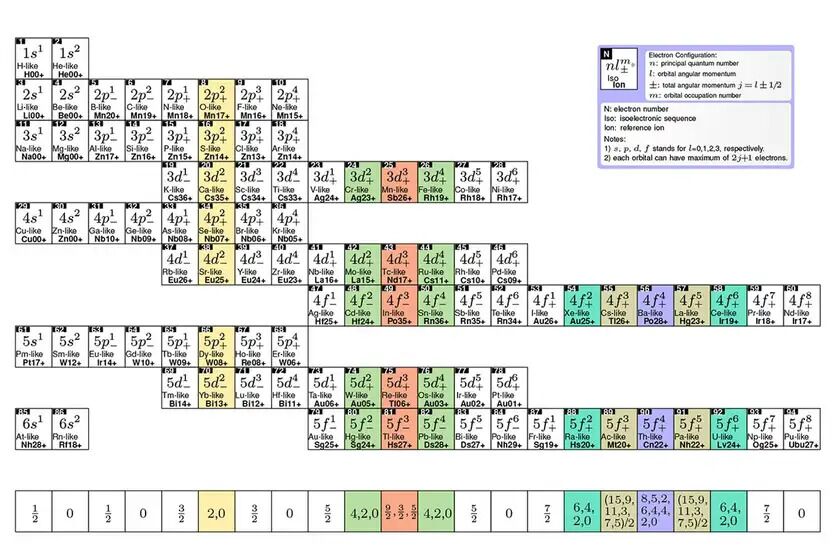
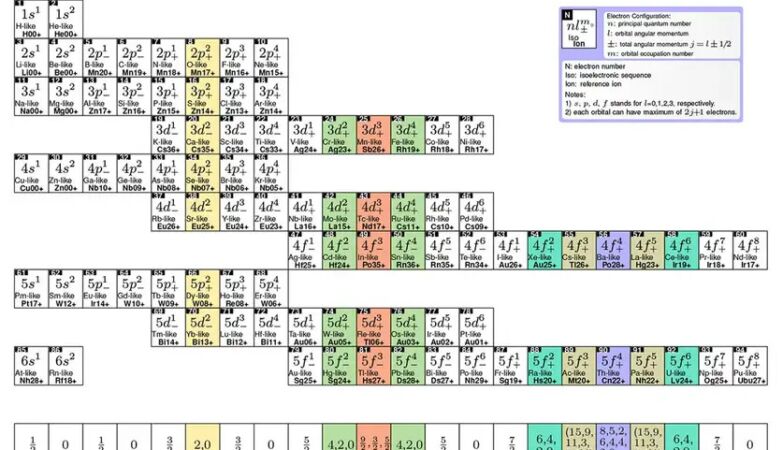
The original periodic table is ordered according to the number of protons that exist in an atom of each element. Instead, Lyu and his colleagues organized the according to the number of electrons in ions. When the atom of an element loses one or more electrons, it can have the same number of electrons as an atom of another element, which means that each table in the table may contain several elements that share the same electron configuration, says Lyu.
The result is a table in which each line represents a layer of electrons and each column represents a subtler. THE layout allowed Lyu and his colleagues to predict what is known as prohibited transitions.
If an atom absorbs energy – for example, when colliding with another atom – the electrons may move from one layer or submitted to another. According to quantum theory, some of these transitions are much more likely than others, depending on the layers where electrons start and end.
But there are also rare and unusual transitions that are not strictly impossible, only highly unlikely and slow to occur. These are known as prohibited transitions And, as they take longer, they are very stable, which makes them ideal for projecting optical atomic watches.
Lyu and his colleagues used his table to predict the existence of 700 highly loaded ions that could be used for these transitions in order to create more accurate optical atomic watches.
Mark Leacha chemist who maintains a database online periodic tables and directs the chemical consulting service Meta-synthesisstates that all these prohibited transitions may exist.
Now that the transitions have been theoretically foreseen, says Lyu, it is possible to adjust the energy of an electron beam to collide with atoms and generate the desired high energy ião and keep it in this state forbidden with lasers.
The Ian could then be measured experimentally with conceoscopy to learn more about the energy structure of the electrons that revolve around the nucleus and employee to build even more accurate atomic watches, says Lyu.
These clocks could help in navigation of spacecraft distant from the land, help coordinate satellites, test Albert Einstein Relativity Theory and operate quantum communication networks.
“This is far from the main idea of the periodic table. It is a configuration of highly ionized elements, ”says Guillermo Restrepofrom the Max Planck Institute of Mathematics in the Sciences, in Leipzig, Germany. “But they discovered interesting and prohibited transitions, which opens a New path to improve atomic watcheswhich is really important. ”
Teresa Oliveira Campos, Zap //