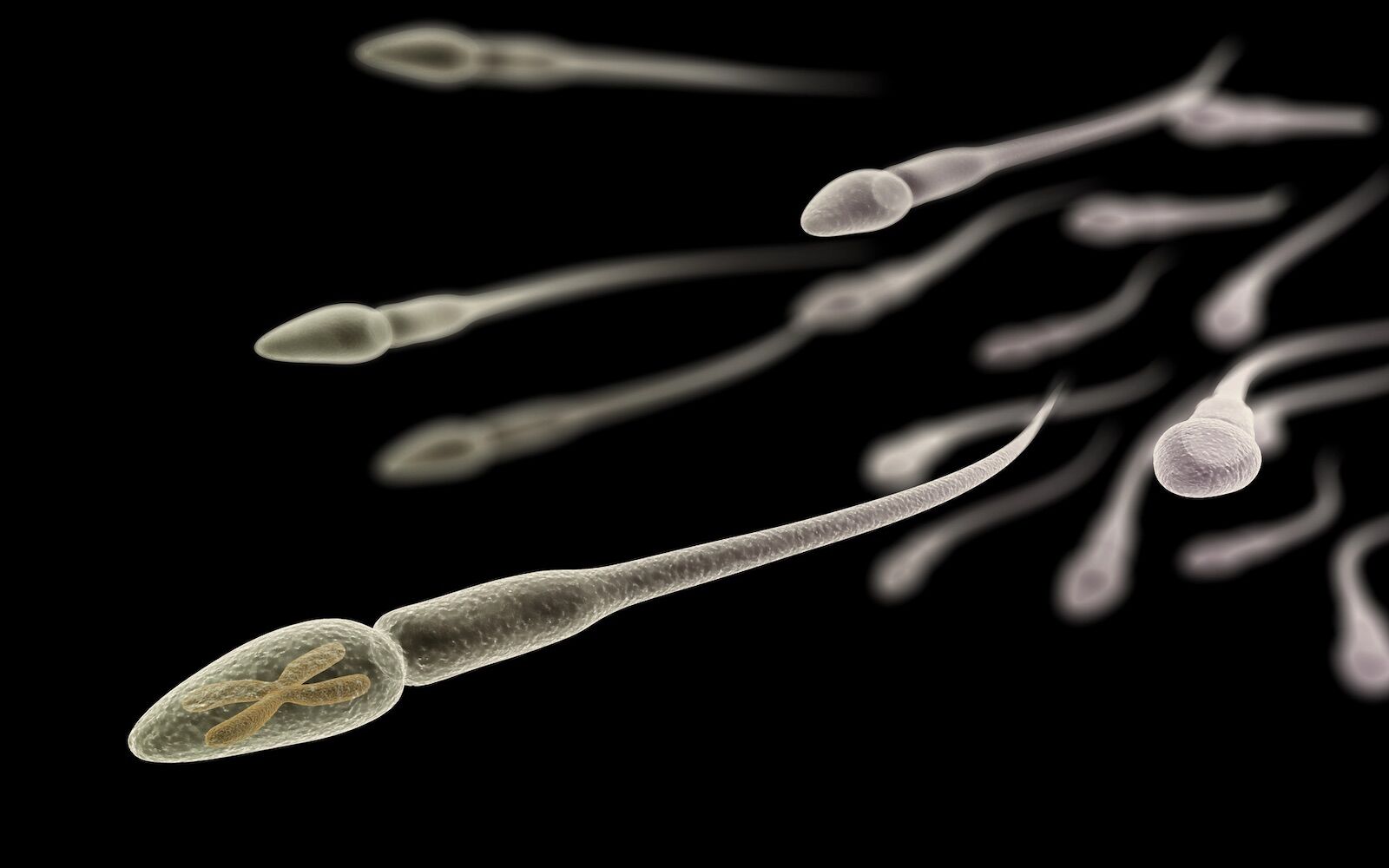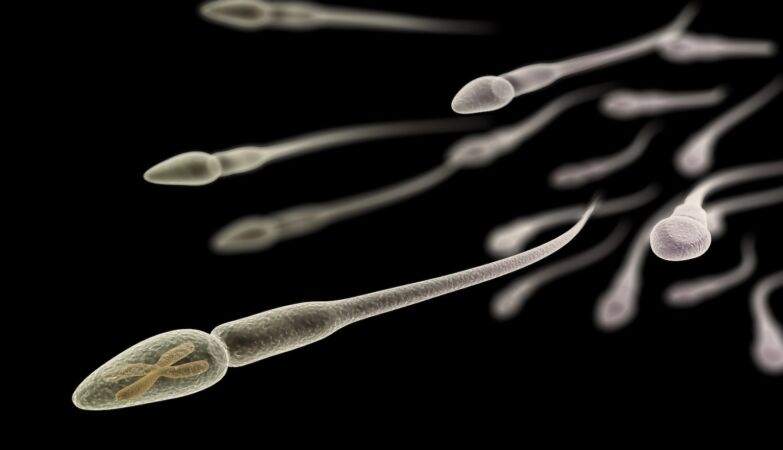
Increasing paternal age has been associated with increased health risks for the next generation, including higher risks of obesity and stillbirths. But what is behind this increased risk remains unknown.
Most of the research into this link focuses on how the DNA inside sperm changes with age. But sperm also transports other compounds, including RNA molecules, vital for protein synthesis.
Now, a new one from the University of Utah (UUH), published this Tuesday in The EMBO Journalshowed that the RNA content of sperm undergoes similar changes over time both, which can lead to a change fast and dramatic in the middle of life.
Furthermore, “old RNA” appears alter cell metabolismpotentially contributing to the health risks associated with having children later in life, say the study authors.
“It’s like finding a molecular clock that advances with age in both mice and humans, suggesting a fundamental and conserved molecular signature of sperm aging,” he says. Qi Chenprofessor of urology and human genetics at the University of Utah and one of the study’s senior authors
“Perhaps this progressive change in length accumulate silentlyuntil triggering the ‘abrupt’ change in the middle of life”, adds Chen.
The importance of RNA
Previous work in Chen’s lab had established that RNA in sperm could be changed by the parent contextincluding diet, and that these changes could have impact on the next generation.
But the types of RNA molecules that seemed to be most important were difficult to detect with conventional techniques. Chen’s team developed an advanced RNA sequencing method called PANDORA-seqto “see” this previously undetectable world of sperm RNA.
When they used this new tool to analyze sperm in mice, researchers identified a pattern that traditional techniques could not detect — a sharp and dramatic transition on sperm RNA content in mice between 50 and 70 weeks of age.
In addition to this “peak aging“, they found what appeared to be a molecular clock.
As males age, the proportions of certain sperm RNAs change progressively — longer fragments become more common, while shorter fragments become less common. When they observed the RNA not human spermresearchers found the same change progressive.
“At first glance, this discovery seems counterintuitive,” says Chen, in a statement published on . “For decades, we have known that as sperm ages, your DNA becomes more fragmented and party. One would expect ARN to follow this pattern. Instead, we found the opposite: sperm-specific RNAs actually become longer with age“.
These changes in RNA can affect the health of offspring in important ways, the results suggest.
When the team introduced a “old RNA” cocktail in mouse embryonic stem cells, which are biologically similar to early embryos, the cells showed changes in gene expression associated with metabolism and neurodegeneration, potentially suggesting a mechanism through which RNA could impact the health of the next generation.
Finding Invisible Patterns
Researchers were only able to detect some of these changes when they looked at RNA just from the sperm head — the part of the sperm that delivers its contents to the egg. The sperm’s long tail contains another RNA that has obscured the pattern until now.
“This change in length of rsRNA it was a unique signspecific to sperm heads. It was obscured by the ‘noisier’ profile of the whole sperm”, explains the corresponding co-author Tong Zhouassociate professor at the University of Nevada, and senior co-author of the paper.
“The sequencing of the sperm head sample was what made this discovery possible,” he adds.
The researchers managed confirm these changes in humans thanks to UUH’s unique clinical and research infrastructure, which directly links basic science laboratories to andrology and patient resources, he says Kenneth Astondirector of the Andrology and IVF Laboratory at the University of Utah and senior co-author of the paper.
“Validating this discovery from mice to humans it was really exciting” says Aston. “Our sperm bank resources at the University of Utah have made this cross-species validation possible.”
“If we can understand the enzymes that drive this change, they could become actionable targets for interventions that potentially improve sperm quality in aging males,” says Chen. “Stay tuned.”