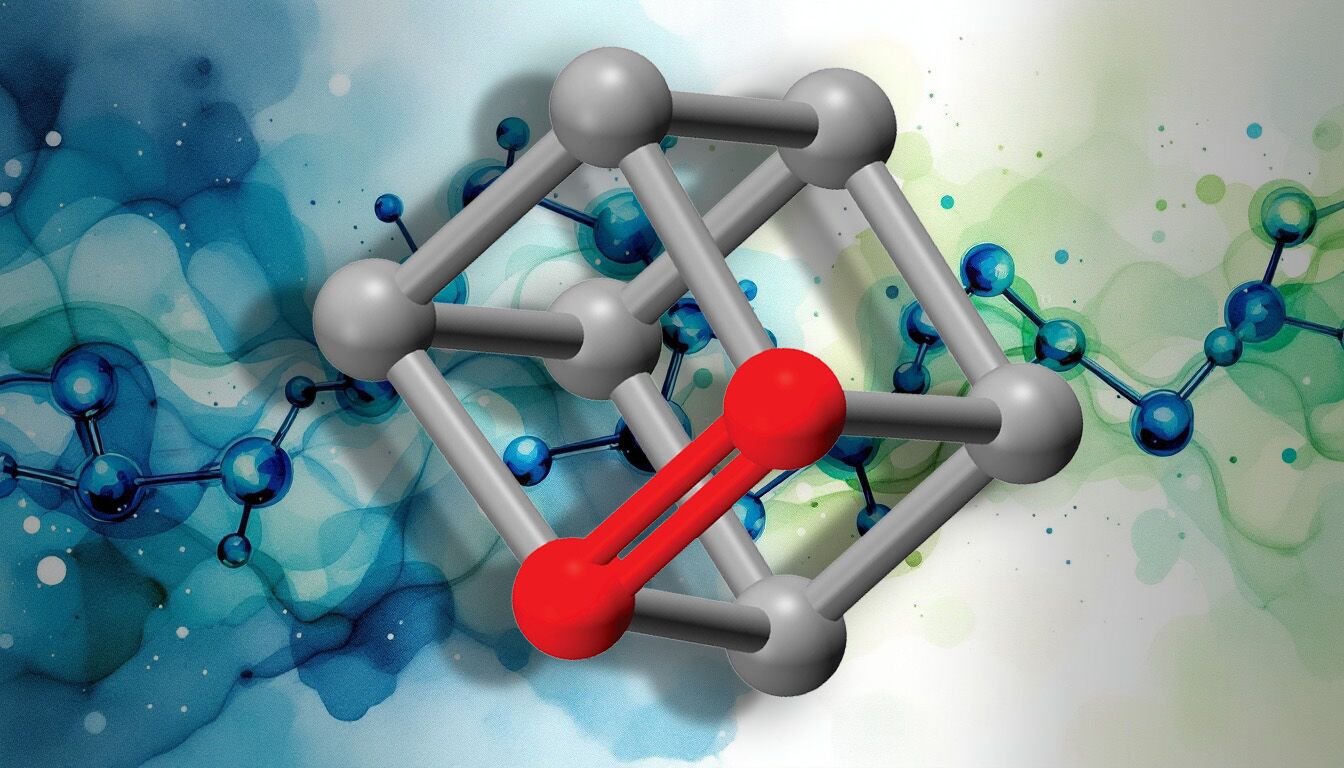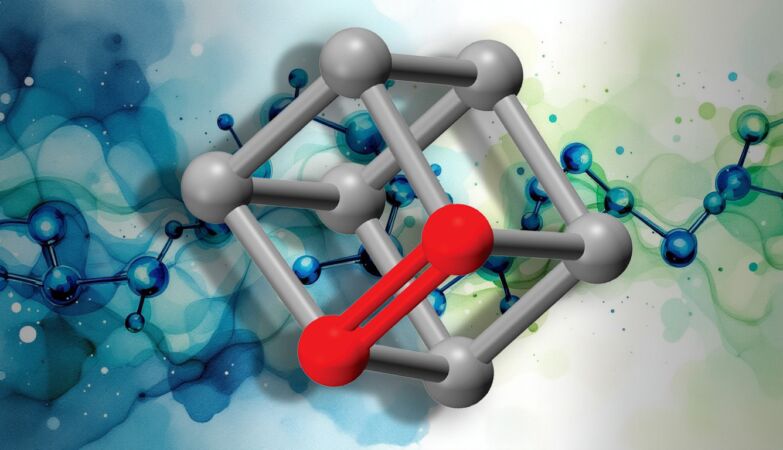
Cubene structure
A team of researchers is rewriting the rules of molecular structure — and the results could reshape the way medicines of the future are manufactured.
UCLA chemists are demonstrating that some of the most famous “regras” of organic chemistry are not as unbreakable as previously thought.
When creating bizarre molecules cage-shaped with distorted double bonds, structures long considered impossiblethe team is pioneering entirely new types of chemistry.
Organic chemistry is based on long-established rules that describe how atoms bond together, how chemical bonds form, and how molecules take shape.
These principles guide how scientists understand reactions and predict molecular behavior. Although many of these rules are treated as absolute truths, researchers at UCLA are demonstrating that chemistry has more flexibility than was believed.
In 2024, a research group led by Neil Gargchemist at UCLA, overturned Bredt’s rulea principle that had been in force for more than a century.
This rule establishes that molecules cannot form a double bond carbon-carbon in the “bridgehead” position (the junction of the rings in a bridged bicyclic molecule), explains .
Based on this revolutionary discoveryGarg’s team has now developed methods for create even stranger structures: cage-shaped molecules known as cubene and quadricyclenewhich contain highly unusual double bonds.
Neil Garg et al
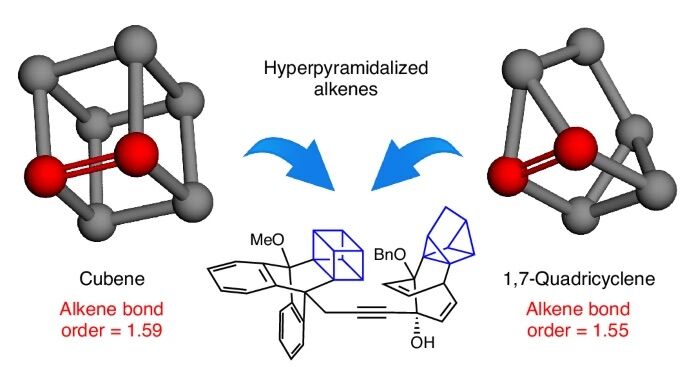
Chemical structure of cubene equadricyclene
In most molecules, atoms linked by a double bond are arranged in a flat arrangement. Neil Garg’s team discovered that this familiar geometry does not apply to cubene and quadricyclene.
Their conclusions, presented in a published Wednesday in the journal Nature Chemistryshow that these molecules force double bonds to adopt distorted three-dimensional shapeswhich expands the range of molecular structures that chemists can imagine and could play an important role in future drug development.
“Decades ago, chemists found solid evidence that we should be able to produce molecules like these, but because we are still so used to thinking about textbook rules about structure, bonding and reactivity in organic chemistry, molecules like cubene and quadricyclene have been avoided,” explains Garg.
After all, almost all of these rules should be treated more as guidelinessays researcher at UCLA.
Organic molecules generally contain three types of bonds: single, double and triple. Carbon-carbon double bonds are called alkenes and have a bond order of 2, which reflects how many pairs of electrons are shared between the bonded atoms. In typical alkenes, the carbons adopt a geometry planar trigonalcreating a flat structure around the double bond.
The molecules studied by Garg’s team, which worked closely with the UCLA computational chemist Ken Houk, behave differently.
Due to their compact and strained shapes, the double bonds in cubene and quadricyclene have a bond order closest to 1.5 than 2. This unusual connection results directly from its three-dimensional geometry.
“Neil’s lab figured out how to make these incredibly distorted molecules, and organic chemists are excited with what can be done with these unique structures”, says Houk.
The discovery comes as scientists actively look for new types of three-dimensional molecules to improve the drug design. Many modern drugs are based on complex forms that interact more precisely with biological targets.
“Producing cubene and quadricyclene was probably considered quite specialized in the 20th century,” Garg said. “But currently we are starting to exhaust the possibilities of regular structures and flatter, and there is a greater need to produce unusual and rigid 3D molecules.”
Garg’s team believes these findings could help pharmaceutical researchers design a new generation of medicineswhich, compared to medicines developed in recent decades, have more complex three-dimensional shapes.
“In my laboratory, three things are most important. One is to advance the foundations of what we know. The second is to do chemistry that might be useful for others and have practical value for society”, says Garg.
“And the third is form all really brilliant people who come to UCLA to get a world-class education and then go into academia, where they continue to discover new things and teach others, or to industry, where they are making medicines or doing other interesting things to benefit our world“.